

The U.S. Food and Drug Administration is pushing back against criticism from medical establishmentarians over the agency's willingness to take a closer look at the health risks posed by antidepressants, specifically selective serotonin reuptake inhibitors, during pregnancy.
Various health organizations, including the American College of Obstetricians and Gynecologists, accused FDA Commissioner Dr. Marty Makary, his agency, and the participants in an expert panel discussion that Makary hosted last month of disseminating "inaccurate" information and of making "outlandish" claims.
'Adolescents exposed to SSRIs in utero exhibited higher anxiety and depression symptoms than unexposed adolescents.'
An FDA spokesperson defended the agency's discussions with experts on the topic, suggesting to Blaze News that the critiques of the agency's expert advisory process were "politically driven."
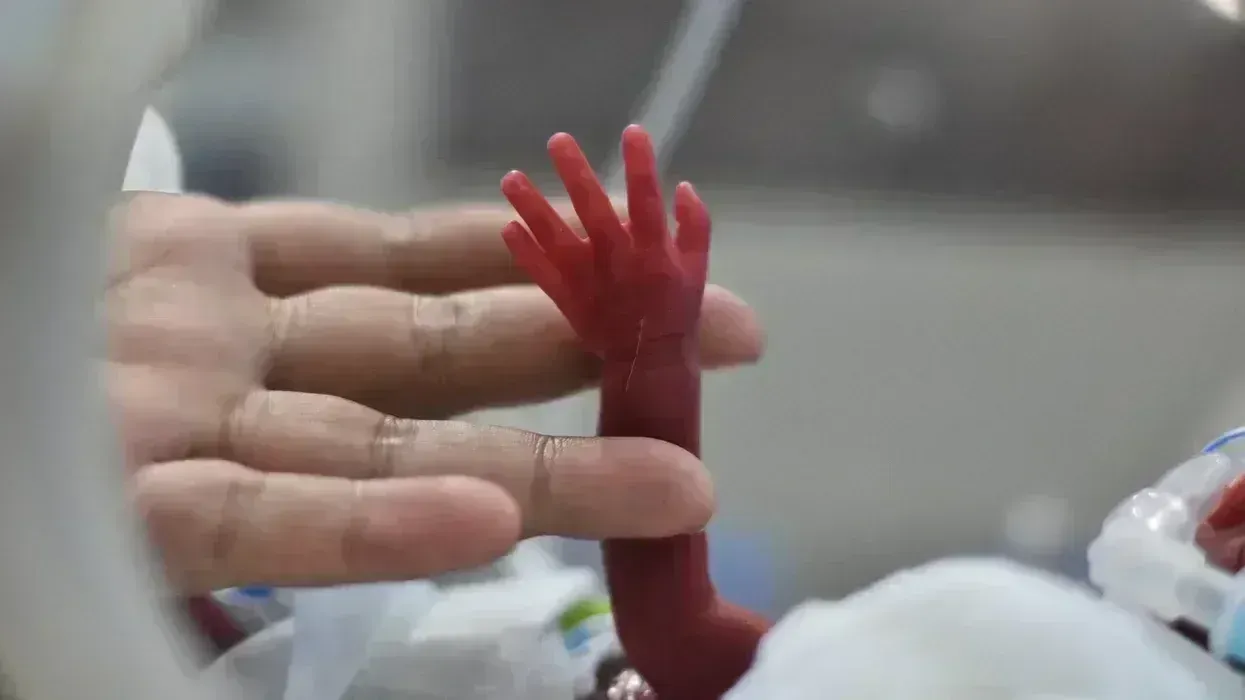
Dr. Jay Gingrich, professor of developmental psychology at the Columbia University Medical Center, noted during the July 21 panel discussion that while expectant mothers suffering depression have long been prescribed SSRIs, it was not until recently that any substantial research was undertaken to determine whether these drugs improved outcomes in the mothers' offspring.
JAMA Medical News confirmed that no randomized clinical trials have been undertaken, due partly to ethical concerns. Despite the absence of such trial data, 6%-8% of pregnant women are reportedly prescribed SSRIs in the United States.
After observing in rodent trials that the mice born of female mice exposed to SSRIs exhibited "stark changes in behavior" and "changes in the brain," Gingrich explored with Finnish researchers whether SSRI exposure in the womb was similarly consequential for human children and found that it was.
RELATED: 'It's immoral': RFK Jr. axes Biden vax reporting requirement, targets doctors' 'hidden incentives'
 Farrukh Saeed/Getty Images
Farrukh Saeed/Getty Images
A study co-authored by Gingrich and published earlier this year in the peer-reviewed journal Nature Communications provided further confirmation of negative impacts, revealing that "adolescents exposed to SSRIs in utero exhibited higher anxiety and depression symptoms than unexposed adolescents and also had greater activation of the amygdala and other limbic structures when processing fearful faces."
The study concluded that "SSRIs are a common therapeutic strategy in perinatal maternal emotional disorders, however the present cross-species data and prior studies on single species indicate that we need more mechanistic understanding of how pharmacological factors like SSRIs impact early brain development and later result in maladaptive behaviors."
'The public needs better information, and the FDA must strengthen the warnings.'
Dr. Adam Urato, chief of maternal-fetal medicine at MetroWest Medical Center in Massachusetts, told his fellow panelists that he has observed in recent years women increasingly taking antidepressants during pregnancy, in many cases thinking SSRIs "don't affect the baby or cause complications."
"These drugs alter the mom’s brain. Why wouldn't they affect the baby’s?" said Urato. "We can see it on prenatal ultrasound. The ultrasound studies show SSRI-exposed fetuses have different movement and behavior patterns. After birth the newborn babies can have jitteriness, breathing difficulties, and higher rates of admission to the neonatal intensive care unit."
"The public needs better information, and the FDA must strengthen the warnings," Urato underscored. "For example, there's currently no warning regarding preterm birth or preeclampsia. The postpartum hemorrhage warning needs to be strengthened. But perhaps the major shortcoming is that the label doesn't make clear that SSRIs alter fetal brain development."
The concerns raised by Gingrich, Urato, and the other panelists evidently ruffled some feathers at organizations that champion the use of SSRIs during pregnancy.
Steven Fleischman, president of the American College of Obstetricians and Gynecologists, rushed to complain, stating shortly after the conclusion of the panel discussion that it "was alarmingly unbalanced and did not adequately acknowledge the harms of untreated perinatal mood disorders in pregnancy," adding, "Robust evidence has shown that SSRIs are safe in pregnancy and that most do not increase the risk of birth defects.
The American College of Obstetricians and Gynecologists' current practice guidelines reportedly recommend SSRIs as a first-line pharmacotherapy for mothers between the time of conception and up until a year after the baby's birth.
Fleischman told JAMA Medical News last week that the panel may "incite fear and cause patients to come to false conclusions that could prevent them from getting the treatment they need."
'Commissioner Makary has an interest in ensuring policies reflect the latest gold-standard science and protect public health.'
Marketa Wills, CEO of the American Psychiatric Association, echoed Fleischman in a July 25 letter to Makary, stating, "We are alarmed and concerned by the misinterpretations and unbalanced viewpoints shared by several of the panelists."
"The inaccurate interpretation of data, and the use of opinion, rather than the years of research on antidepressant medications, will exacerbate stigma and deter pregnant individuals from seeking necessary care," wrote Wills.
In addition to stating that "the overall evidence suggests that individuals can and should take SSRIs prior to or during pregnancy, when they are clinically indicated for treatment," Wills claimed that "recent meta-analyses have found no association between prenatal SSRI exposure and overall risk of birth defects."
The Society for Maternal-Fetal Medicine similarly complained, suggesting that the panelists made "unsubstantiated and inaccurate claims."
RELATED: RFK Jr. pulls plug on mRNA jabs because they 'pose more risks than benefits'
 Dobrila Vignjevic/Getty Images stock photo
Dobrila Vignjevic/Getty Images stock photo
Other groups similarly outraged by the discussion of possible downsides to drugs characterized as safe and effective include Postpartum Support International, the National Curriculum in Reproductive Psychiatry, and the Massachusetts General Hospital for Women's Mental Health.
An FDA spokesperson told Blaze News, "The claim that the FDA’s expert advisory process is 'one-sided' or politically driven is insulting to the independent scientists, clinicians, and researchers who dedicate their expertise to these panels."
"FDA expert panels are roundtable discussions with independent panels of scientific experts that will review the latest scientific evidence, evaluate potential health risks, explore safer alternatives, and individual experts may offer their recommendations for regulatory action," continued the spokesperson. "This initiative is part of the FDA’s broader efforts to apply rigorous, evidence-based standards to ingredient safety and modernize regulatory oversight, thoroughly considering evolving science and consumer health."
The spokesperson noted that "Commissioner Makary has an interest in ensuring policies reflect the latest gold-standard science and protect public health" and stated that suggesting "his engagement on women’s health signals a desire to manipulate outcomes is politically motivated and undermines the serious work being done to improve care for millions of women."
Like Blaze News? Bypass the censors, sign up for our newsletters, and get stories like this direct to your inbox. Sign up here!
.png)
 2 hours ago
3
2 hours ago
3












 English (US)
English (US)